สล็อต เว็บตรง แตกง่าย มาแรงที่สุดชั้น 1 ในช่วงเวลานี้ แหล่งรวมเกมสล็อตจากค่ายชั้นสุดยอด เล่นเหมาะนี่กับ Cafe444 !
สวัสดีค่ะทุกท่าน คนใดกันที่กำลังมองหาเว็บไซต์สล็อตออนไลน์ วันนี้เราอยากมาชี้แนะ cafe44 ของทางเราซึ่งเป็นเว็บสล็อตที่สะสมเกมสล็อตออนไลน์จากค่าย PG ซึ่งเป็นหนึ่งในผู้พัฒนาเกมที่ได้รับความนิยมสูงสุดในแวดวงสล็อตออนไลน์กันนะคะ
 ไม่เพียงแค่เท่านี้ทางพวกเรายังจะมาบอกข้อดีของเว็บสล็อตออนไลน์ Cafe444 กันอีกด้วยจ้ะ ว่าทำไมถึงกำลังเป็นที่นิยมในปี 2024 นี้ มาฝ่าไปพร้อมได้เลยค่ะ
ไม่เพียงแค่เท่านี้ทางพวกเรายังจะมาบอกข้อดีของเว็บสล็อตออนไลน์ Cafe444 กันอีกด้วยจ้ะ ว่าทำไมถึงกำลังเป็นที่นิยมในปี 2024 นี้ มาฝ่าไปพร้อมได้เลยค่ะ
pgslot เว็บสล็อตออนไลน์จากค่าย pg slot ดียังไง ? ทำไมกำลังเป็นที่ชื่นชอบปัจจุบันนี้ ?
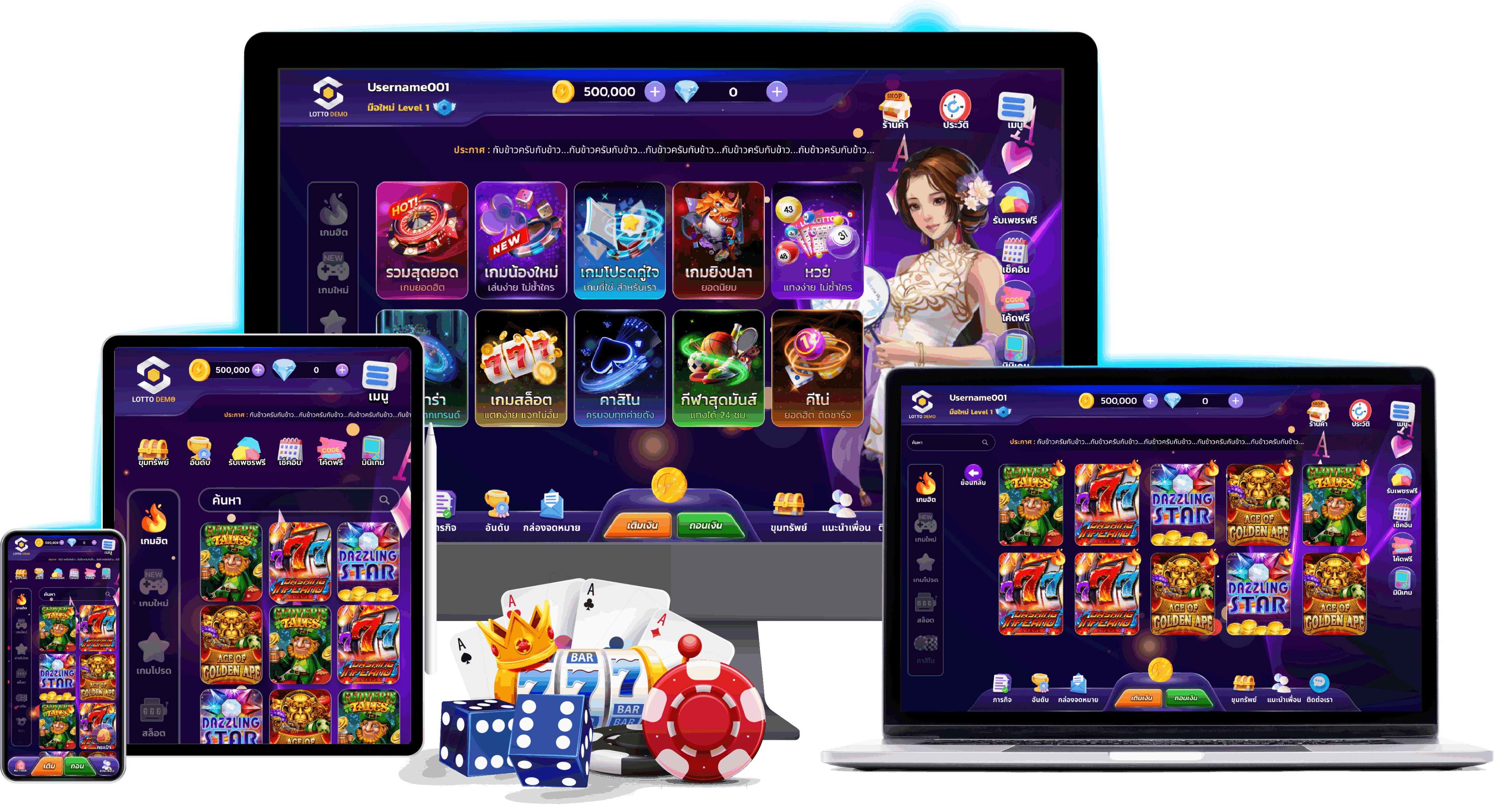
เว็บไซต์สล็อต Cafe444 พวกเราให้บริการเกมสล็อตออนไลน์ที่ครบวงจร และเกมอื่นๆอีกมากมาย ไม่ใช่แค่เพียงสล็อต แม้กระนั้นในเว็บไซต์พวกเรายังบริการเกม บาคาร่า , สล็อต แทงหวย , เกมยิงปลา , ป๊อกกระดอน , คีโน่ แล้วก็เกมแผนภูมิอีกด้วย ที่นี่มีทุกอย่างที่คุณอยาก!
นอกเหนือจากการที่จะมีเกมสล็อตหรือคาสิโนที่นานาประการและน่าเร้าใจแล้ว พวกเรายังมีโปรโมชั่นที่น่าสนใจมากเพื่อเพิ่มโอกาสในการชนะและก็ทำให้ประสบการณ์การเล่นเกมของผู้เล่นดีอย่างขึ้นไปอีก! ไม่ว่าคุณจะเป็นพวกใหม่หรือผู้เล่นประจำ พวกเรามีโปรโมชั่นที่เหมาะสมกับทุกท่านแล้วก็ทางพวกเราบริการทั่วถึงทุกผู้เล่นอย่างไม่ต้องสงสัย !
บริการเกมคาสิโนทุกรูปแบบ มีให้เลือกเล่นนานาประการ พร้อมที่เว็บเดียวที่ Cafe444 !
ทางพวกเราได้เก็บรวบรวมเกมยอดนิยมของผู้เล่นต่างๆโดยทางพวกเราจะย้ำที่เกมที่มีคุณภาพ หากเป็นเกมสล็อตทางพวกเราก็จะนำเกมสล็อตจากค่ายๆดังๆสุดยอดมาแค่นั้น อาทิเช่นจากค่าย pgslot
เรามีเกมคาสิโนออนไลน์นานัปการให้คุณเลือกเล่น ไม่ว่าจะเป็น
 ● เกมสล็อตออนไลน์ – เกมสล็อตออนไลน์เป็นเลิศในเกมคาสิโนยอดนิยมสูงสุดในโลกของการพนันออนไลน์ เนื่องจากว่ามีกติกาที่เข้าใจง่าย และไม่จำเป็นต้องใช้อุบายซับซ้อน และก็กราฟฟิคของเกมที่กราฟฟิคสวย และธีมต่างๆให้ได้เลือกเล่นและก็เพลินใจ ทำให้เหมาะกับทั้งผู้เล่นมือใหม่และผู้เล่น cafe44 ที่มีประสบการณ์
● เกมสล็อตออนไลน์ – เกมสล็อตออนไลน์เป็นเลิศในเกมคาสิโนยอดนิยมสูงสุดในโลกของการพนันออนไลน์ เนื่องจากว่ามีกติกาที่เข้าใจง่าย และไม่จำเป็นต้องใช้อุบายซับซ้อน และก็กราฟฟิคของเกมที่กราฟฟิคสวย และธีมต่างๆให้ได้เลือกเล่นและก็เพลินใจ ทำให้เหมาะกับทั้งผู้เล่นมือใหม่และผู้เล่น cafe44 ที่มีประสบการณ์
● บาคาร่าออนไลน์ – บาคาร่าออนไลน์เป็นเยี่ยมในเกมไพ่ยอดนิยมอย่างมากในคาสิโนทั่วโลก และก็เมื่อมาอยู่ในแบบอย่างออนไลน์ ก็ยังคงความง่ายๆและก็เสน่ห์ที่ทำให้ผู้เล่นหลายล้านคนเผลอไผล
● เกมยิงปลา – เกมยิงปลาเป็นหนึ่งในเกมคาสิโนออนไลน์ที่เข้ามาครอบครองใจผู้เล่นมากไม่น้อยเลยทีเดียวด้วยการผสมผสานระหว่างกราฟิกที่สวย การเล่นเกมที่ตื่นเต้น แล้วก็จังหวะสำหรับการชนะรางวัล ด้วยความธรรมดาและก็ความสนุกสนานร่าเริง ไม่น่าฉงนใจที่เกมยิงปลาได้แปลงเป็นที่นิยมอย่างรวดเร็วในกลุ่มผู้เล่นคาสิโนออนไลน์
● แทงหวย – การแทงหวยออนไลน์ยอดเยี่ยมในรูปแบบการพนันยอดนิยมอย่างมากในกลุ่มผู้เล่นทั้งโลก เพราะว่ามันสบาย ง่ายสุดๆ และสามารถเข้าถึงได้จากทุกๆที่ ลอตเตอรี่ออนไลน์มีหลายแบบ ตั้งแต่หวยรัฐบาล หวย ไปจนถึงสลากกินแบ่งต่างชาติ อย่างเช่น ลอตเตอรี่ลาว ลอตเตอรี่ฮานอย รวมทั้งสลากกินแบ่งยี่กี ฯลฯ ซึ่งทางพวกเรามีลอตเตอรี่มากมายให้ทุกท่านได้เลือกเล่น
● ป๊อกกระเด้ง – ป๊อกเด้งเป็นเกมไพ่ที่มีต้นกำเนิดในประเทศไทยและมีชื่อเสียงอย่างกว้างขวางในหมู่ผู้เล่นคาสิโนในทวีปเอเชีย โดยเฉพาะในกลุ่มชุมชนไทย ป๊อกเด้งเป็นเกมที่เล่นง่ายแล้วก็มีจังหวะการเล่นที่รวดเร็วทันใจ ทำให้เป็นที่ชื่นชอบมากๆทางพวกเราจึงมีการนำเกมไพ่ป๊อกเด้งมาบริการกับทางผู้เล่นด้วย
● คีโน่ – คีโน่เป็นเกมที่มีลักษณะคล้ายกับการเล่นลอตเตอรี่และก็บิงโก ซึ่งมีที่มาจากจีนรวมทั้งเป็นที่นิยมในคาสิโนทั้งโลก รวมถึงคาสิโนออนไลน์ เกมนี้เรียบง่ายแม้กระนั้นมอบโอกาสสำหรับการชนะรางวัลที่นานาประการ ทำให้ผู้เล่นหลายคนหันมาสนใจในขณะนี้
● เกมกราฟ – หรือ “Crash games” เป็นเกมที่ผสมระหว่างการเดิมพันแล้วก็ส่วนประกอบของกราฟิกเพื่อสร้างประสบการณ์การเล่นเกมที่น่าเร้าใจและก็เต็มไปด้วยการกระตุ้นทางอารมณ์ ในเกมนี้ ผู้เล่นจึงควรทำพนันแล้วก็สังเกตดูกราฟที่เพิ่มขึ้นอย่างต่อเนื่อง โดยมีโอกาสถอนเงินก่อนที่จะแผนภูมิจะ “พัง” หรือ “แตก”
ด้วยความมากมายหลากหลายของเกม สล็อต มีคุณภาพทำให้เว็บไซต์ cafe44 มีชื่อเสียงในแวดวงสล็อตออนไลน์ในปี 2024 นี้
ระบบฝาก-ถอน ออโต้ ไม่ต้องรอคอยคิวแอดไม่น ฝาก-ถอน ได้ข้างใน 5 นาทีเท่านั้น!
ระบบการฝาก-ถอนออโต้ในเว็บ สล็อต ของทางพวกเราเช็ดสร้างออกแบบมาเพื่อให้ผู้เล่นได้รับประสบการณ์ที่ยอดเยี่ยมในการทำธุรกรรมด้านการเงิน ซึ่งอีกทั้งรวดเร็วรวมทั้งไม่เป็นอันตราย นี่เป็นจุดเด่นสำคัญๆของระบบการฝาก-ถอนออโต้ของทางเรา
ความรวดเร็ว ทำงานฝาก-ถอน ด้านใน 5 นาที
การฝากและเบิกเงินด้วยระบบออโต้ทำให้ผู้เล่นเกม cafe44 ไม่ต้องรอนาน ไม่ต้องรอคอยฝาก-ถอน ต่อคิวกับทางเจ้าหน้าที่แอดมินผู้เล่นสามารถที่จะทำงานได้ด้วยตัวเองเพื่อย่นเวลาของทางผู้เล่นเองรวมทั้งกระบวนการทำงานนั้นก็ไม่ต้องการที่จะอยากเลยเพียงแค่ไม่กี่ขั้นตอนเพียงแค่นั้น
ความปลอดภัยที่เข้มงวด
ระบบธุรกรรมของทางพวกเรามากับมาตรการความปลอดภัยระดับสูง เงินไม่มีสูญหาย ข้อมูลถูกเก็บโดยสวัสดิภาพ และทางเรามีการตรวจเช็คระบบความปลอดภัยอยู่เสมอ
ระบบการฝาก-ถอนออโต้ของ cafe44 จัดว่าจุดขายที่สำคัญของทางเราก็ว่าได้ โดยไม่เพียงแค่ความสบายและเร็วทันใจ แม้กระนั้นยังรักษามาตรฐานความปลอดภัยในระดับสูงให้กับผู้เล่นอีกด้วย!
มาร่วมเป็นส่วนหนึ่งของครอบครัว pgslot และก็เริ่มการเสี่ยงภัยในโลกของเกมสล็อตออนไลน์กับทางเราได้เลย สมัครวันนี้ทางพวกเรามีโปรโมชั่นแจกเครดิตฟรีเยอะสำหรับผู้เล่นใหม่อีกด้วยค่ะ ^^
Casino สล็อต Cafe444.me 11 มีนาคม Jeanna เกมpgสนุกๆ cafe44ไม่จำกัดยอดโอน Top 61
ขอขอบคุณอ้างอิงจาก cafe44


 PGSLOT จัดเต็มความรื่นเริงใจเต็มรูปแบบ slot หาง่าย สล็อตแจกเครดิตฟรีปังๆ รับประกันความแตกง่าย ทดลองเลย!
PGSLOT จัดเต็มความรื่นเริงใจเต็มรูปแบบ slot หาง่าย สล็อตแจกเครดิตฟรีปังๆ รับประกันความแตกง่าย ทดลองเลย! 3 สิ่งที่คุณต้องดูซิเลือกเกมสล็อตออนไลน์สักเกมจากสล็อต pg
3 สิ่งที่คุณต้องดูซิเลือกเกมสล็อตออนไลน์สักเกมจากสล็อต pg
 รับซื้อทะเบียนสวยต่างจังหวัดอยากขายทะเบียนรถ ขายทะเบียนรถ tabiengod.com 23 เมษา 24 Bryant ซื้อป้ายทะเบียนรถใหม่ทะเบียนสวยราคาถูก ขายทะเบียนรถเลขทะเบียนรถสวย ความหมายดี Top 22
รับซื้อทะเบียนสวยต่างจังหวัดอยากขายทะเบียนรถ ขายทะเบียนรถ tabiengod.com 23 เมษา 24 Bryant ซื้อป้ายทะเบียนรถใหม่ทะเบียนสวยราคาถูก ขายทะเบียนรถเลขทะเบียนรถสวย ความหมายดี Top 22 • สำหรับสายบาคาร่าพวกเราก็มีมาแนะนำ ไม่ได้มีให้เล่นแต่ว่าสล็อตอย่างเดียว เพราะว่าเมืองไทย มีตัวแทนของค่าย sexy baccarat ของแท้ มาให้ทุกคนได้เล่นกันอย่างเต็มลิมิตด้วยชื่อเว็บไซต์ sexyauto168 นั่นเองแรง
• สำหรับสายบาคาร่าพวกเราก็มีมาแนะนำ ไม่ได้มีให้เล่นแต่ว่าสล็อตอย่างเดียว เพราะว่าเมืองไทย มีตัวแทนของค่าย sexy baccarat ของแท้ มาให้ทุกคนได้เล่นกันอย่างเต็มลิมิตด้วยชื่อเว็บไซต์ sexyauto168 นั่นเองแรง ดาวน์โหลดpg slot pgslot Pgslotth.asia 23 มีนาคม Vickie เกมpgสนุกๆ slogแจกโบนัสทุกซีซั่น Top 61
ดาวน์โหลดpg slot pgslot Pgslotth.asia 23 มีนาคม Vickie เกมpgสนุกๆ slogแจกโบนัสทุกซีซั่น Top 61 ขอขอบคุณwebsite
ขอขอบคุณwebsite  DOOMOVIE ดูหนังออนไลน์กับเว็บหนังออนไลน์ที่ยอดเยี่ยม ไม่พลาดทุกความเบิกบานใจแบบจัดเต็ม มาดูหนังกัน!
DOOMOVIE ดูหนังออนไลน์กับเว็บหนังออนไลน์ที่ยอดเยี่ยม ไม่พลาดทุกความเบิกบานใจแบบจัดเต็ม มาดูหนังกัน! ตามหาเว็บดูหนังผ่านอินเตอร์เน็ต ตามหาเรา ดูหนังออนไลน์ สิขอรับ! พวกเราเป็นเว็บดูหนังผ่านอินเตอร์เน็ตที่พร้อมมอบความรื่นเริงใจรวมทั้งประสบการณ์การหนังออนไลน์ที่ไม่เหมือนใคร เราจัดเต็มทั้งยังหนังเด็ด ซีรีส์ดัง และก็อีกเยอะแยะ ให้ท่านสนุกสนานกับการรับชมความเพลิดเพลินได้แบบไม่สิ้นสุด หนังใหม่ชนโรง หนังเก่าที่คิดถึง หรือหนังหาดูยาก ก็สามารถมาดูกับเราได้เลยจ๊ะนะครับ ไม่ต้องสมัครเป็นสมาชิก สามารถมองได้ทันที บอกเลยว่า ฟินต้อนรับวันหยุดแน่ๆ
ตามหาเว็บดูหนังผ่านอินเตอร์เน็ต ตามหาเรา ดูหนังออนไลน์ สิขอรับ! พวกเราเป็นเว็บดูหนังผ่านอินเตอร์เน็ตที่พร้อมมอบความรื่นเริงใจรวมทั้งประสบการณ์การหนังออนไลน์ที่ไม่เหมือนใคร เราจัดเต็มทั้งยังหนังเด็ด ซีรีส์ดัง และก็อีกเยอะแยะ ให้ท่านสนุกสนานกับการรับชมความเพลิดเพลินได้แบบไม่สิ้นสุด หนังใหม่ชนโรง หนังเก่าที่คิดถึง หรือหนังหาดูยาก ก็สามารถมาดูกับเราได้เลยจ๊ะนะครับ ไม่ต้องสมัครเป็นสมาชิก สามารถมองได้ทันที บอกเลยว่า ฟินต้อนรับวันหยุดแน่ๆ หนังใหม่ชนโรงก็มองได้โดยทันที! ดูหนังใหม่ชนโรงได้ก่อนคนใด คลิกเลยที่ DOOMOVIE แค่นั้น!
หนังใหม่ชนโรงก็มองได้โดยทันที! ดูหนังใหม่ชนโรงได้ก่อนคนใด คลิกเลยที่ DOOMOVIE แค่นั้น!


 2. โปรโมชั่นทุกยอดฝาก 10%
2. โปรโมชั่นทุกยอดฝาก 10%

 HYPERX
HYPERX  1. ระบบหลังบ้านดีเยี่ยม
1. ระบบหลังบ้านดีเยี่ยม
 pgslot โปรโมชั่นสุดคุ้ม ที่จะทำให้คุณได้บันเทิงใจเต็มแม็กซ์เยอะขึ้นเรื่อยๆ
pgslot โปรโมชั่นสุดคุ้ม ที่จะทำให้คุณได้บันเทิงใจเต็มแม็กซ์เยอะขึ้นเรื่อยๆ ทั้งสิ้นนี้คือโปรโมชั่นสล็อตที่เว็บของพวกเรา ถ้าหากคุณสนใจอยากรับโปรโมชั่น pgslot ที่เว็บของพวกเราได้เลย
ทั้งสิ้นนี้คือโปรโมชั่นสล็อตที่เว็บของพวกเรา ถ้าหากคุณสนใจอยากรับโปรโมชั่น pgslot ที่เว็บของพวกเราได้เลย สล็อตเว็บตรง สนุก บันเทิงใจเร้าใจ แหล่งพนันที่ใหญ่ที่สุดอยู่ตรงนี้แล้ว
สล็อตเว็บตรง สนุก บันเทิงใจเร้าใจ แหล่งพนันที่ใหญ่ที่สุดอยู่ตรงนี้แล้ว
 1. ระบบรักษาความปลอดภัยในด้านข้อมูลส่วนตัว หรือการเก็บข้อมูลในตอนลงทะเบียนหรือการยื่นข้อมูลเพิ่มเติมนอกเหนือจากนี้สำหรับการรับเครดิตฟรีโปรโมชั่นหรือโบนัสต่างๆนั่นเองแรง
1. ระบบรักษาความปลอดภัยในด้านข้อมูลส่วนตัว หรือการเก็บข้อมูลในตอนลงทะเบียนหรือการยื่นข้อมูลเพิ่มเติมนอกเหนือจากนี้สำหรับการรับเครดิตฟรีโปรโมชั่นหรือโบนัสต่างๆนั่นเองแรง 2. ระบบรักษาความปลอดภัยสำหรับเพื่อการโอนเข้าออกเงินระหว่างเว็บของพวกเรากับบัญชีของทุกท่าน จะปลอดภัยในระดับสูงสุด เนื่องจากด้วยความที่เราเปลี่ยนระบบมาให้ใช้ระบบ AI ทั้งหมด 100% ดังนั้น ข้อมูลส่วนนี้ที่เป็นส่วนที่บอบบางที่สุด เราได้กระทำจัดแจงดูแลอย่างดี โดยที่บุคลากรชั้นสูงก็ยังเข้าไม่ถึง
2. ระบบรักษาความปลอดภัยสำหรับเพื่อการโอนเข้าออกเงินระหว่างเว็บของพวกเรากับบัญชีของทุกท่าน จะปลอดภัยในระดับสูงสุด เนื่องจากด้วยความที่เราเปลี่ยนระบบมาให้ใช้ระบบ AI ทั้งหมด 100% ดังนั้น ข้อมูลส่วนนี้ที่เป็นส่วนที่บอบบางที่สุด เราได้กระทำจัดแจงดูแลอย่างดี โดยที่บุคลากรชั้นสูงก็ยังเข้าไม่ถึง

 ขอขอบคุณwebsite
ขอขอบคุณwebsite 


 pgslot เกมสล็อตแตกหนักมากกว่า 200 เกม เล่นได้ไม่จำกัด สนุกสนานได้ไม่ยั้ง
pgslot เกมสล็อตแตกหนักมากกว่า 200 เกม เล่นได้ไม่จำกัด สนุกสนานได้ไม่ยั้ง แอพสล็อตPG slot funny18.in 16 มกราคม 2024 Silas จ่ายจริง pgslotจ่ายโบนัสเยอะ Top 49
แอพสล็อตPG slot funny18.in 16 มกราคม 2024 Silas จ่ายจริง pgslotจ่ายโบนัสเยอะ Top 49
 • ที่พวกเรายังอยู่จนถึงทุกวันนี้ภายหลังเปิดให้บริการกว่าสิบปี คุณมีความคิดว่าเพราะอะไรกันล่ะ? แน่ๆว่ามันควรจะเป็นเพราะเหตุว่าความชอบธรรมและไม่โกงของพวกเรา ระบบของเราที่บรรเจิด ทำให้ลูกค้าหนาแน่นรวมทั้งวางใจวางใจเรา กระทั่งทำเราให้มาถึงจุดจุดนี้ได้ จำต้องขอขอบคุณมากลูกค้าหน้าเก่าและก็คนใหม่ และก็คนที่กำลังสนใจอีกด้วยแรง สล็อตxo slot จัดให้!
• ที่พวกเรายังอยู่จนถึงทุกวันนี้ภายหลังเปิดให้บริการกว่าสิบปี คุณมีความคิดว่าเพราะอะไรกันล่ะ? แน่ๆว่ามันควรจะเป็นเพราะเหตุว่าความชอบธรรมและไม่โกงของพวกเรา ระบบของเราที่บรรเจิด ทำให้ลูกค้าหนาแน่นรวมทั้งวางใจวางใจเรา กระทั่งทำเราให้มาถึงจุดจุดนี้ได้ จำต้องขอขอบคุณมากลูกค้าหน้าเก่าและก็คนใหม่ และก็คนที่กำลังสนใจอีกด้วยแรง สล็อตxo slot จัดให้! • เรื่องของความปลอดภัยแบบสุดจัดปลัดบอก ไม่มีวันที่ผู้ใดกันแน่จะเข้ามาแตะระบบของเราได้ และก็พวกเราเองก็ไม่อาจจะไปแก้ระบบอะไรมั่วๆได้ ด้วยเหตุว่ามันเขียนอยู่ในหนังสือสัญญาระหว่าง เว็บสล็อตออนไลน์ของพวกเรารวมทั้งค่ายสล็อตออนไลน์ระดับโลกว่า ห้ามปรับแต่งระบบทุกกรณี โดยยิ่งไปกว่านั้นระบบอัตราการสุ่มเดาของเกมส์นั่นเองจ้า
• เรื่องของความปลอดภัยแบบสุดจัดปลัดบอก ไม่มีวันที่ผู้ใดกันแน่จะเข้ามาแตะระบบของเราได้ และก็พวกเราเองก็ไม่อาจจะไปแก้ระบบอะไรมั่วๆได้ ด้วยเหตุว่ามันเขียนอยู่ในหนังสือสัญญาระหว่าง เว็บสล็อตออนไลน์ของพวกเรารวมทั้งค่ายสล็อตออนไลน์ระดับโลกว่า ห้ามปรับแต่งระบบทุกกรณี โดยยิ่งไปกว่านั้นระบบอัตราการสุ่มเดาของเกมส์นั่นเองจ้า

 Fullslotpg ที่สุดของเว็บไซต์สล็อตออนไลน์ไม่ผ่านเอเย่นต์ รับรองหน้าแรกของ Google สมัครเลย!
Fullslotpg ที่สุดของเว็บไซต์สล็อตออนไลน์ไม่ผ่านเอเย่นต์ รับรองหน้าแรกของ Google สมัครเลย! ถ้าคุณกำลังตามหาเว็บไซต์สล็อตออนไลน์ไม่ผ่านเอเย่นต์อยู่ล่ะก็ ลองใช้บริการของพวกเรา
ถ้าคุณกำลังตามหาเว็บไซต์สล็อตออนไลน์ไม่ผ่านเอเย่นต์อยู่ล่ะก็ ลองใช้บริการของพวกเรา  เก็บรวบรวมทุกกิจกรรมจากเรา FULLSLOTPG จัดเต็มทุกความพิเศษได้ทุกวี่วัน ลองเลย!
เก็บรวบรวมทุกกิจกรรมจากเรา FULLSLOTPG จัดเต็มทุกความพิเศษได้ทุกวี่วัน ลองเลย!


 สามารถสร้างรายได้ให้แก่สมาชิกเก่าทุกคนด้วยการนำโปรโมชั่นเหล่านั้นมาประกอบร่วมด้วยกับโปรโมชันที่ให้เลือกมาก สำหรับ
สามารถสร้างรายได้ให้แก่สมาชิกเก่าทุกคนด้วยการนำโปรโมชั่นเหล่านั้นมาประกอบร่วมด้วยกับโปรโมชันที่ให้เลือกมาก สำหรับ

 หลักแสน หลักล้าน หรือหลักสิบล้าน ก็สามารถถอนได้แบบชิวๆรับสิทธิพิเศษดีๆจำนวนมากจากโปรโมชั่นและก็กิจกรรมต่างๆของเรา เพื่อให้คุณไม่พลาดทุกจังหวะสำหรับการได้กำไร เราพร้อมจัดเต็มสิ่งที่ดีที่สุดให้กับคุณ ถ้าเกิดคุณพร้อมแล้วที่จะรับสิทธิพิเศษดีๆเหล่านี้ สมัครสมาชิกแล้วมารับกันได้เลยนะครับ ยังมีสิทธิพิเศษดีๆอีกเพียบ สมัครเลย!
หลักแสน หลักล้าน หรือหลักสิบล้าน ก็สามารถถอนได้แบบชิวๆรับสิทธิพิเศษดีๆจำนวนมากจากโปรโมชั่นและก็กิจกรรมต่างๆของเรา เพื่อให้คุณไม่พลาดทุกจังหวะสำหรับการได้กำไร เราพร้อมจัดเต็มสิ่งที่ดีที่สุดให้กับคุณ ถ้าเกิดคุณพร้อมแล้วที่จะรับสิทธิพิเศษดีๆเหล่านี้ สมัครสมาชิกแล้วมารับกันได้เลยนะครับ ยังมีสิทธิพิเศษดีๆอีกเพียบ สมัครเลย! 5 เหตุผลที่คุณควรเล่นสล็อตออนไลน์จากค่าย PGSLOT pg slot เว็บตรง กับเรา มีระบบระเบียบทดลองเล่นด้วยนะ!
5 เหตุผลที่คุณควรเล่นสล็อตออนไลน์จากค่าย PGSLOT pg slot เว็บตรง กับเรา มีระบบระเบียบทดลองเล่นด้วยนะ!



